Research Highlight: Norbert Kruse
Catalysis & Kinetics
Catalytic and Kinetic Studies with Syngas (CO H2)
Formation of hydrocarbons with terminal functionalization (alcohols, aldehydes, olefins) from syngas is being studied over Fe-, Co- and CoCu-based catalysts. The terminal alcohol or combined terminal alcohol/olefin selectivities usually reach values over 60% and occasionally up to 95% over ‘CoCuMn’ catalysts (see reference 4). The Anderson-Schulz-Flory chain-lengthening probabilities for these products are higher than 0.6, but usually below 0.9, so as to optimize the yield of the C8–C14 slate which is used as feedstock for plasticizers, lubricants, or detergents.
Tuning the chemical composition in Co- and CoCu-based catalysts allows varying the hydrocarbon chain length between long and short. ‘CoCuNb’ catalysts may produce terminal alcohols with a selectivity of ~55 % while the combined terminal alcohols/alkenes selectivities may achieve values above 70%. The chain lengthening probabilities are lower than for ‘CoCuMn’, usually around 0.3 to 0.4, so the C2-C5 slate is favored here and therefore fulfills the needs as a fuel additive. More recent results demonstrate the possibility of optimizing the long-chain aldehyde rather than terminal alcohol/olefin production. It is most intriguing that the early product fractions of CO hydrogenations (methane, methanol, ethane etc.) are strongly suppressed in this case.
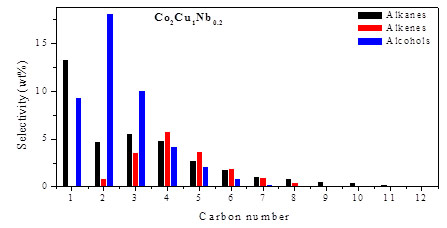
Fe(0) monodisperse nanoparticles with varying size are being investigated to demonstrate the structure sensitivity of the CO hydrogenation. Such particles, once they are intercalated into a mesoporous MCF-17 host, turn out to be highly active. Their methane and short-chain olefin selectivities are highest for the smallest nanoparticles (1.8 nm). The selectivity to long-chain C5+ terminal olefins and oxygenates increases with the particle size (see image below). The Anderson-Schulz-Flory chain lengthening probabilities also increase with particle size for C2+ paraffins and C4+ olefins.

To receive mechanistic information about the CO hydrogenation, Chemical Transient Kinetics (CTK) is applied (see references 7 and 10). The method provides quantitative information about the construction of the catalytically active phase until the steady state is reached. To study the build-up, gas flows are switched fast from adsorption (H2/He) to reaction conditions (H2+CO/Ar). Inverting the procedure provides information on how fast the steady state fades away (see image below). Quantification is possible by solving the time-dependent mass balance. The elemental composition of the catalyst surface during reaction can be determined quantitatively: “surface atom counting.”
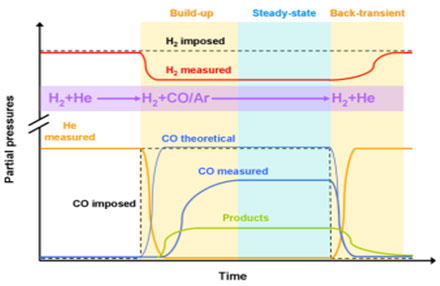
CTK for the CO hydrogenation over Co-based catalysts shows a nonlinear dependence of the Anderson-Schulz-Flory chain lengthening probability with increasing C-atoms during build-up. Plotting against the CO pressure yields a linear relationship which is a strong indication for a CO insertion mechanism to be in operation for chain lengthening.
Characterization of Catalysts for Syngas Reactions
To characterize catalysts for CO hydrogenation to functionalized hydrocarbons, Atom Probe Tomography (APT) is being applied besides High-resolution Transmission Electron Microscopy (HRTEM). APT provides 3D atom-by-atom imaging of a single catalyst grain via field evaporation. By applying a position sensitive detector to a time-of-flight mass spectrometer, field-evaporated ions are sorted according to their mass-to-charge ratio and original position at the catalyst surface. A 3D reconstruction allows the entire catalyst grain to be visualized and every atom to be chemically identified.
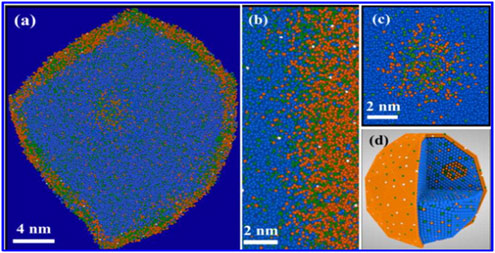
The image shows a single CoCuMn catalyst nanoparticle (Co atoms are depicted in blue, Cu atoms in orange, Mn atoms in green, and O in white. A faceted core–shell structure that contained intracore clusters is observed. (b) An enlarged view from a 5 nm thick slice of the data at the core–shell interface. Oxygen (white) was distributed throughout the core–shell interface. (c) intracore clusters contain a high concentration of Cu and Mn. (d) A 3D sectional view of the element distribution. Cooperation with prof. S. Ringer at University of Sydney (see reference 4).
HRTEM studies are being performed with Fe(0) nanoparticles prepared via colloidal recipes (cooperation with prof. B. Chaudret at the University of Toulouse, France). The particles are monodisperse and either directly deposited on a copper grid or intercalated into an MCF-17 silica foam.

Imaging of Catalytic Reactions using Video- Field Ion Microscopy (FIM)
The combustion of hydrogen to water in fuel cells is mimicked in the video-field ion microscope with nanoscale lateral resolution (see reference 11). The video below shows the catalytic reaction to be strongly non-linear and to merge into self-sustained rate oscillations. Only a single nanosized catalyst particle, Rh metal in this case, is used to demonstrate the sequence of patterns to comprise oxygen islands to form in the center of the surface and to extend anisotropically to the crystal periphery. Once the entire surface is crowded by oxygen atoms, a fast clean-off reaction with hydrogen leads to water formation. Then the catalytic cycle starts again. Rate oscillations with high robustness are presently also investigated in the catalytic reduction of NOx with hydrogen. The phenomenology is reminiscent of a “nanoclock” since the oscillation periods may become very regular over extended periods of time.
Rate oscillations during the O2-H2 reaction on Rh: “The Nanoclock”
Cross-section of the reaction pattern is about 20nm; Oscillation period: t ~ 40 s; Frame rate: x4; Video taken at CPMCT of the Université Libre de Bruxelles
Selection of recently published papers in context with the research highlights
- Yizhi Xiang, Roland Barbosa, and Norbert Kruse. “Higher Alcohols through CO Hydrogenation over CoCu Catalysts: Influence of Precursor Activation” ACS Catal., 2014, 4 (8), pp 2792–2800.
- Chenakin, S. P., N. Kruse, M. A. Vasylyev, and I. N. Makeeva. “XPS/ToF-SIMS Characterization of TiO2 Supported Au Nanoparticles: Effect of Catalytic CO Oxidation.” Metal Physics and Advanced Technologies, 2014, 36(5), pp. 597—611.
- Barroo, C., Y. De Decker, T. Visart de Bocarmé, and N. Kruse. “Complex Oscillation Patterns During the Catalytic Hydrogenation of NO2 Over Platinum Nano-sized Crystallites.” J. Phys. Chem. C, 2014, 118 (13), pp 6839–6846. DOI: 10.1021/jp412506e
- Xiang, Y., V.Chitry, P.Liddicoat, P.Felfer, J.Cairney, S. Ringer, and N. Kruse. “Long-Chain Terminal Alcohols Through Catalytic CO Hydrogenation.” J. Am. Chem. Soc, 135 (19), (2013) 7114–7117. DOI: 10.1021/ja402512r
- Chenakin, S. P., G. Melaet, R. Szukiewicz and N. Kruse. “XPS Study of the Surface Chemical State of a Pd/(SiO2+TiO2) Catalyst After Methane Oxidation and SO2 Treatment.” J. Catal., 312 (2014) 1-11. DOI: 10.1016/j.jcat.2014.01.008
- Devred, F., P. Dulgheru, and N. Kruse. “Elemental Steps in Heterogeneous Catalysis” Comprehensive Inorganic Chemistry (Elsevier), Encyclopedia, 2012, chapter 7.02 DOI:10.1016/B978-0-08-097774-4.00703-8
- Schweicher, J., A. Bundhoo, and N. Kruse. “Hydrocarbon Chain Lengthening in Catalytic CO Hydrogenation: Evidence for a CO-Insertion Mechanism.” J. Am. Chem. Soc. 134 (2012) 16135-16138. DOI: 10.1021/ja3068484
- Vesselli, E., J. Schweicher, A. Bundhoo, A. Frennet and N. Kruse. “Catalytic CO2 Hydrogenation on Nickel: Novel Insight by Chemical Transient Kinetics.” J. Phys. Chem. C 115 (2011) 1255–1260. DOI: 10.1021/jp106551r
- McEwen, J.-S., Y. De Decker, P. Gaspard, C. Barroo, T. Visart de Bocarmé and N. Kruse. “Catalytic Reduction of NO2 with Hydrogen on Pt Field Emitter Tips: Kinetic Instabilities on the Nanoscale.” Langmuir 26 (2010) 16381-16391. DOI: 10.1021/la102003x
- Schweicher, J., A. Bundhoo, A. Frennet, N. Kruse, H. Daly and F.C. Meunier. “DRIFT/MS Studies during Chemical Transients and SSITKA of the CO/H2 Reaction over Co-MgO Catalysts.” J. Phys. Chem. C 114 (2010) 2248-2255. DOI: 10.1021/jp909754w
- McEwen, J.-S., P. Gaspard, T. Visart de Bocarmé, and N. Kruse. “Nanometric Chemical Clocks.” Proc. Natl. Acad. Sci. U. S. A. 106, (2009) 3006-3010. DOI: 10.1073/pnas.0811941106